Hi, Greetings from FWQRC……….
This blog is related to how block chain contributes to drug supply management
WHAT IS BLOCK CHAIN?
Block chain, or a distributed ledger, is a way of organisation information is a way that gives all appropriate parties access to the information they need and keeps that information secure from people who should not see it
In terms of quality, block chains can ensure that every part of a supply chain can have assurance that the materials & products moving through it have reached a particular standard passed checks and compiled with necessary regulations
In its simple form,block chain is information that is shared across a group of computers so that if one person updates that information others are able to see it
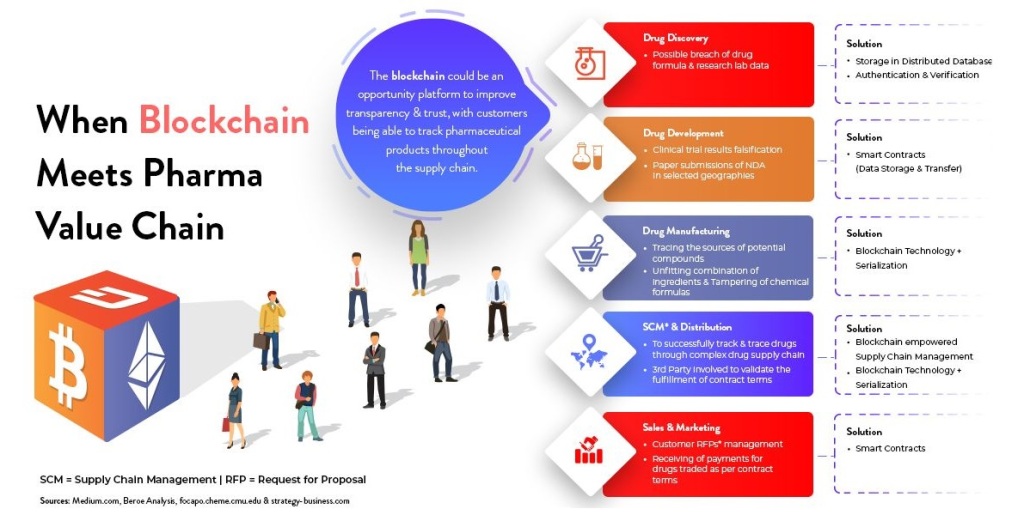
HOW BLOCK CHAIN CONTRIBUTES TO DRUG SUPPLY-CHAIN MANAGEMENT
Imagine a simple supply chain: Company A produces raw material;company B makes it into a product,while company C sells it. With block chain,company A can alert company B & company C of changes in supply chain-such as overproduction-that they can then use to moderate their manufacturing process. Company B could extend their working hours and sales strategy, while company C could plan a marketing campaign to move the extra product
Sharing decentralized information in this way means that business relationships will become much more flexible,benefiting the participants and requiring no outside help. This can be a highly effective means of self regulation
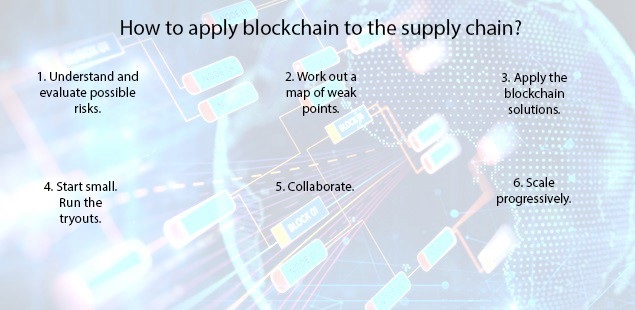
HOW BUSINESSES ARE USING BLOCK CHAIN IN THEIR SUPPLY CHAIN:
Global research firm, Gartner, predicts that by 2023,some 30% of manaufacturing complanies with revenue of more than $5bn will be using block chain to drive down costs and improve tracebility and transperancy
Block chain as a strategy will force companies to look beyond the boundaries of their own firm & establish shared process and consensus mechanisms with their supply chain partners
CHALLENGES OF THE MODEL
- The benefits are great,but they may come at a cost
- Block chain represents a challenge for businesses “Companies that have an ageing information technology infrastructure will struggle to interact effectively with digitally native companies
- Tech companies have a responsibility to make the user experience as easy & seamless as possible for everyone in the supply chain
Thank you for viewing FWQRC blogs……………………