
AUDITS



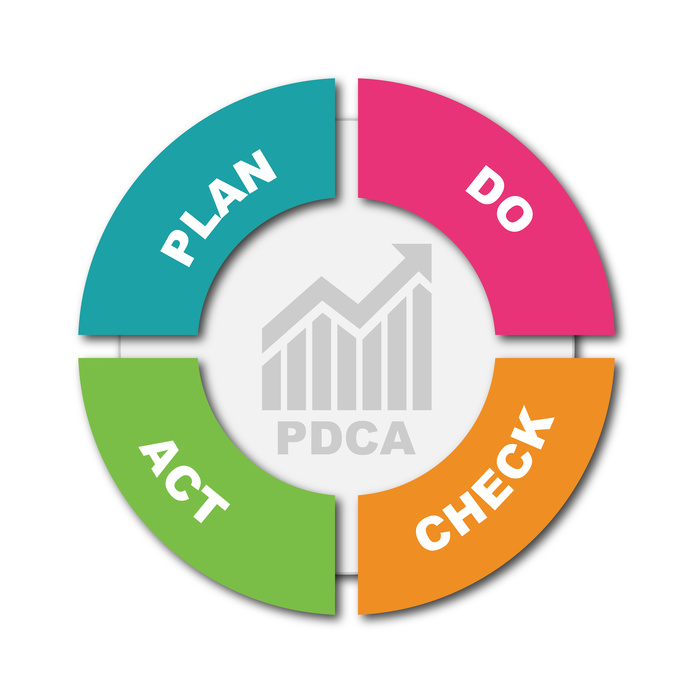 Auditing for GMP is specifically designed to address the challenges of GMP auditing for the pharmaceutical industry and present the basic competencies required to effectively perform the auditor’s assigned responsibilities and contribute to the improvement of auditor performance within a regulated industry.
Auditing for GMP is specifically designed to address the challenges of GMP auditing for the pharmaceutical industry and present the basic competencies required to effectively perform the auditor’s assigned responsibilities and contribute to the improvement of auditor performance within a regulated industry. Qualified Person (QP) from FWQRC™ facilitates for third party GMP inspections.
Qualified Person (QP) from FWQRC™ facilitates for third party GMP inspections.