Hi Everyone
Good evening………………………
Today We are going to discuss on eCTD
CTD is an ICH standard that FDA adopted in a consensus process, as a member of ICH, together with other member regions, Europe and Japan Currently global format for regulatory submissions Consistent data organization Method to electronically transfer product information and data Collection of electronic files organized according to guidelines defining file format, folder/files naming convention, document specifications etc. Applies to all NDAs, ANDAs, BLAs, INDs and master files.

The eCTD challenge In the US, eCTD-only NDAs, BLAs and INDs are accepted – no paper necessary eCTD plus paper still needed for Medical authorities in EU Paper is still the official archival copy of the EU MA EU wants eCTD as preferred format for all Marketing Authorisation Applications(MAAs) and variations Only eCTD for MA for all EU members states by 1 Jan 2010 Health Canada wants eCTD format on CD/DVD plus paper
NEED FOR ELECTRONIC SUBMISSIONS
Designed with consideration that facilitate Creation Review Assists project management and information management Life cycle management (the history of a product application) Archiving Drug development planning
Current Status of US eCTD Submissions FDA Office of Chief Information Officer Quarterly briefing, 12 Dec 2008 During the period 2005 to 2008, eCTD submission volume grew at a compounded annual growth rate of approximately 300%.
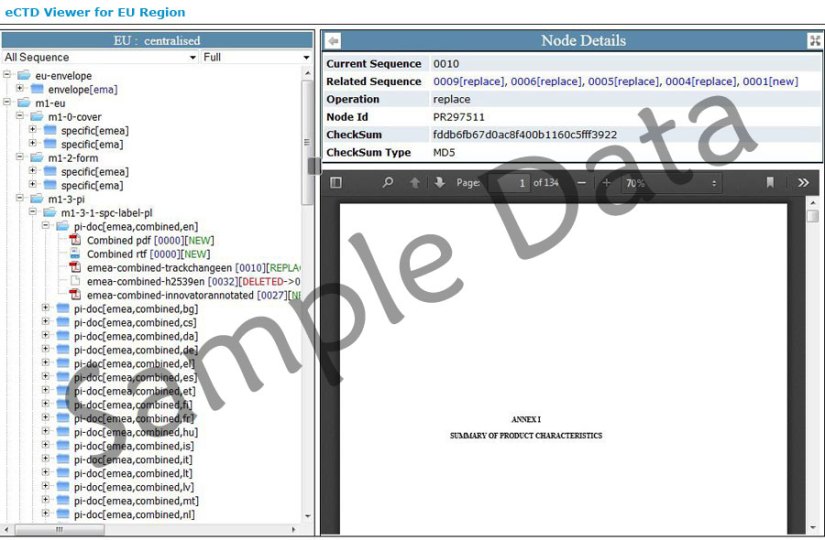
Basics of eCTD The ICH M4 Expert Working Group (EWG) has defined the Common Technical Document (CTD). The ICH M2 EWG has defined, in the current document, the specification for the Electronic Common Technical Document (eCTD). There are 5 modules in the submission report. A hierarchical cabinet/folder structure containing the electronic documents (PDF). An XML backbone that provides a structure to display the PDF documents in the eCTD format (eCTD viewer)
References
- CDER Contact for information on eCTD submissions esub@fda.hhs.gov
- CDER Contact for information on SDTM submission cder-edata@fda.hhs.gov Electronic
- Regulatory Submissions and Review website http://www.fda.gov/cder/regulatory/ersr/default.htm
- International Conference on Harmonization http://www.ich.org
- All FDA Guidances on Electronic Submissions http://www.fda.gov/cder/guidance/index.htm#electronic_submissions


It’s good!!!
LikeLike
Thank you
LikeLike
Great
LikeLike
It is very useful
LikeLike